Evidence That Skeletal Muscles Modulate HDL-Cholesterol in Metabolic Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric, Body Composition and Laboratory Parameters
2.3. Statistical Analysis
3. Results
3.1. Study Population
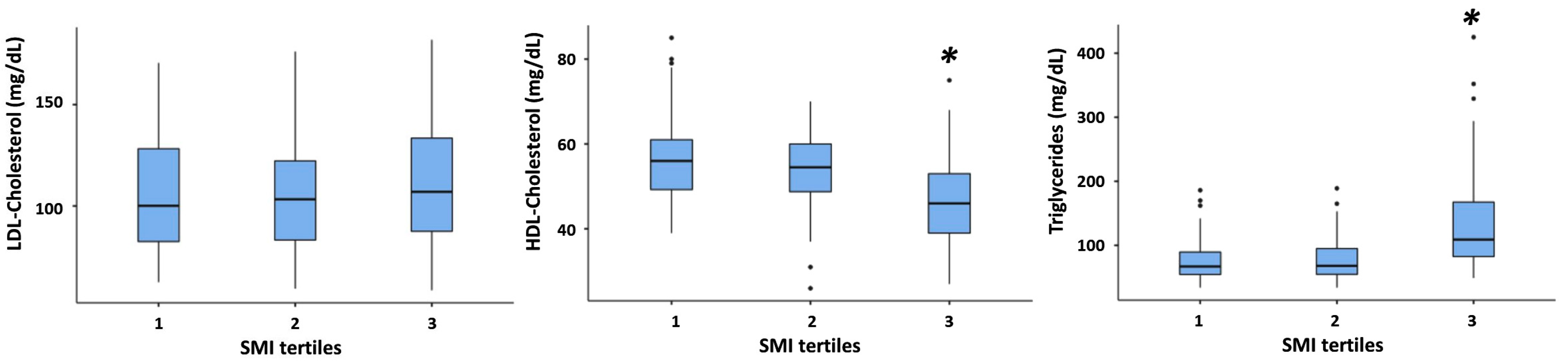
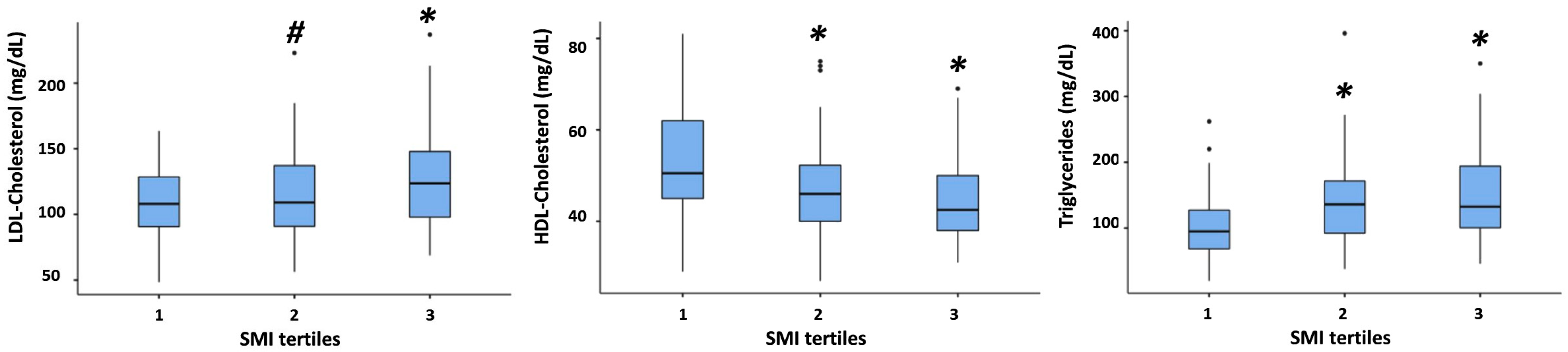
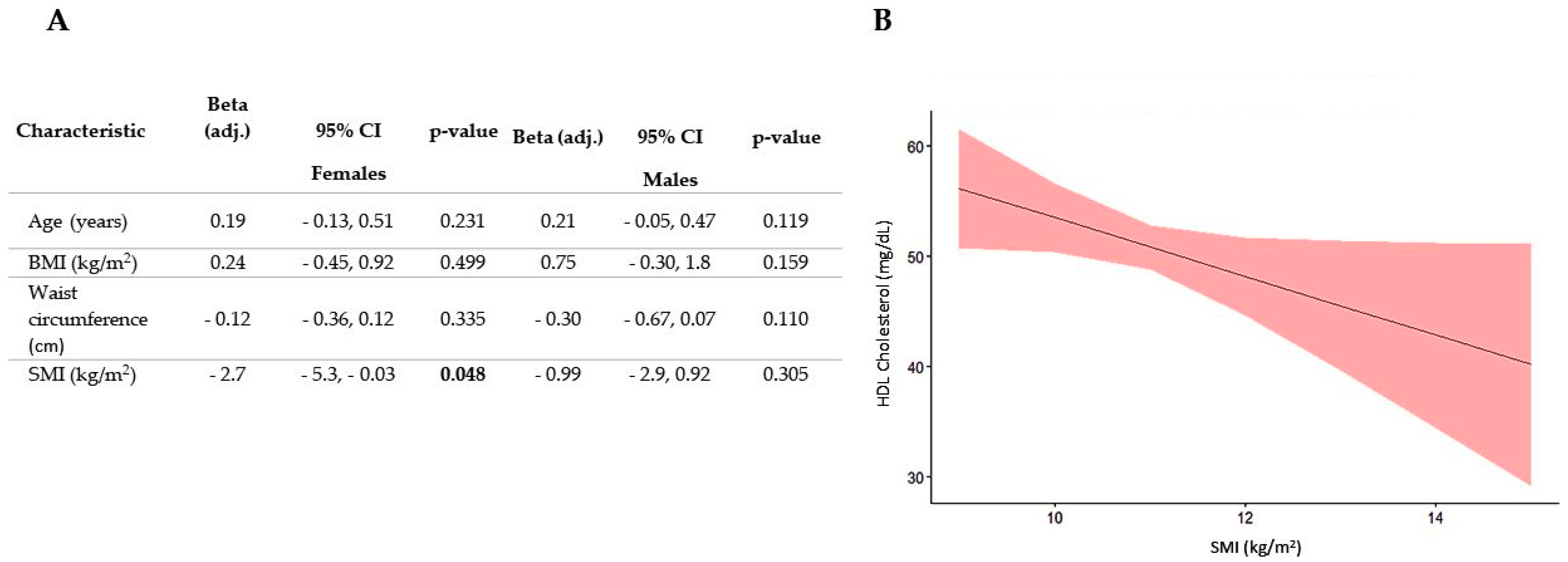
3.2. Association between SMI and All Plasma Lipid Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP III | Adult Treatment Panel III |
| BIA | Bioelectrical Impedance Analysis |
| BIVA | Bioelectrical Vector Analysis |
| BMI | Body Mass Index |
| CETP | Cholesteryl ester transfer protein |
| CT | Computed Tomography |
| ECW | Extracellular Water |
| FFM | Fat Free Mass |
| FFMI | Fat Free Mass Index |
| FM | Fat Mass |
| FMI | Fat Mass Index |
| Gly | Glycemia |
| GOT | Glutamic-Oxalacetic Transaminase |
| GPT | Glutamic Pyruvic Transaminase |
| HDL-C | High-Density Lipoprotein-Cholesterol |
| HOMA index | Homeostasis Model Assessment index |
| IMAT | Intermuscular Adipose Tissue |
| IRQ | Interquartile range |
| LDL-C | Low Density Lipoprotein-Cholesterol |
| LPL | Lipoprotein Lipase |
| MRI | Magnetic Resonance Imaging |
| MS | Metabolic Syndrome |
| NCEP | National Cholesterol Education Program |
| NW | Normal weight |
| Ob | Obese |
| OW | Overweight |
| Q1 | first quartile |
| Q3 | third quartile |
| Rs | Resistance |
| SM | Skeletal Muscle |
| SMI | Skeletal Muscle Index |
| TBW | Total body water |
| TBW | Total Body Water |
| TG | Triglycerides |
| TRL | Triglyceride-rich lipoproteins |
| VLDL | Very Low-Density Lipoproteins |
| WHO | World Health Organization |
| Xc | Reactance |
References
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Contraction-Induced Myokine Production and Release: Is Skeletal Muscle an Endocrine Organ? Exerc. Sport Sci. Rev. 2005, 33, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef]
- Wolfe, R.R. The Underappreciated Role of Muscle in Health and Disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and Tissue Sites of Non-Insulin- and Insulin-Mediated Glucose Uptake in Humans. Am. J. Physiol. Endocrinol. Metab. 1988, 255, E769–E774. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Gunnarsson, R.; Björkman, O.; Olsson, M.; Wahren, J. Effects of Insulin on Peripheral and Splanchnic Glucose Metabolism in Noninsulin-Dependent (Type II) Diabetes Mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal Muscle Metabolism Is a Major Determinant of Resting Energy Expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Bjorkman, O.; Reichard, G.A.; Pilo, A.; Olsson, M.; Wahren, J.; DeFronzo, R.A. The Disposal of an Oral Glucose Load in Healthy Subjects: A Quantitative Study. Diabetes 1985, 34, 580–588. [Google Scholar] [CrossRef]
- Ranallo, R.F.; Rhodes, E.C. Lipid Metabolism during Exercise. Sports Med. 1998, 26, 29–42. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Kaijser, L.; Rössner, S. Removal of exogenous triglycerides in human forearm muscle and subcutaneous tissue. J. Intern. Med. 1975, 197, 289–294. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle Wasting in Disease: Molecular Mechanisms and Promising Therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Nair, K.S. Sarcopenia of Aging and Its Metabolic Impact. Curr. Top. Dev. Biol. 2005, 68, 123–148. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.-W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between Sarcopenia Level and Metabolic Syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, T.; Methenitis, S.; Panagiotakos, D.B. The Emerging Role of Skeletal Muscle as a Modulator of Lipid Profile the Role of Exercise and Nutrition. Lipids Health Dis. 2022, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- World Health Organization (WHO). ProMED-Mail Website. 2016. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 March 2020).
- Guida, B.; Cataldi, M.; Maresca, I.D.; Germanò, R.; Trio, R.; Nastasi, A.M.; Federico, S.; Memoli, A.; Apicella, L.; Memoli, B.; et al. Dietary Intake as a Link between Obesity, Systemic Inflammation, and the Assumption of Multiple Cardiovascular and Antidiabetic Drugs in Renal Transplant Recipients. Biomed. Res. Int. 2013, 2013, 363728. [Google Scholar] [CrossRef]
- Kotler, D.; Burastero, S.; Wang, J.; Pierson, R. Prediction of Body Cell Mass, Fat-Free Mass, and Total Body Water with Bioelectrical Impedance Analysis: Effects of Race, Sex, and Disease. Am. J. Clin. Nutr. 1996, 64, 489S–497S. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of Skeletal Muscle Mass by Bioelectrical Impedance Analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Piccoli, A. Bioelectric impedance measurement for fluid status assessment. Contrib. Nephrol. 2010, 164, 143–152. [Google Scholar] [CrossRef]
- Lagacé, J.; Brochu, M.; Dionne, I.J. A Counterintuitive perspective for the role of fat-free mass in metabolic health. J. Cachexia Sarcopenia Muscle 2020, 11, 343–347. [Google Scholar] [CrossRef]
- Brochu, M.; Mathieu, M.; Karelis, A.D.; Doucet, É.; Lavoie, M.; Garrel, D.; Rabasa-Lhoret, R. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: A Monet Study. Obesity 2008, 16, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.W.; Kemper, H.C.; Mellenbergh, G.J.; Van Mechelen, W. Relation between the longitudinal development of lipoprotein levels and biological parameters during adolescence and young adulthood in Amsterdam, The Netherlands. J. Epidemiol. Community Health 1996, 50, 505–511. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Lee, R.C.; Capristo, E.; Deckelbaum, R.J.; Heymsfield, S.B. An Independent, inverse association of high-density-lipoprotein-cholesterol concentration with nonadipose body mass. Am. J. Clin. Nutr. 1999, 69, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.M.; Rogers, N.L.; Remsberg, K.E.; Sun, S.S.; Chumlea, W.C.; Demerath, E.W.; Czerwinski, S.A.; Towne, B.; Siervogel, R.M. lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: The Fels Longitudinal Study. Int. J. Obes. 2006, 30, 251–260. [Google Scholar] [CrossRef]
- Duran, I.; Martakis, K.; Schafmeyer, L.; Jackels, M.; Rehberg, M.; Schoenau, E. Inverse association of high-density lipoprotein cholesterol concentration with muscle mass in children. Child. Obes. 2019, 15, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Donkers, J.H.; Fredrix, E.W.; Boekhoudt, P. Energy intake, physical activity and body weight: A simulation model. Br. J. Nutr. 1995, 73, 337–347. [Google Scholar] [CrossRef]
- Marron, M.M.; Allison, M.; Kanaya, A.M.; Larsen, B.; Wood, A.C.; Herrington, D.; Greenland, P.; Miljkovic, I. Associations between lipoprotein subfractions and area and density of abdominal muscle and intermuscular adipose tissue: The multi-ethnic study of atherosclerosis. Front. Physiol. 2021, 12, 713048. [Google Scholar] [CrossRef]
- Sinha, R.; Dufour, S.; Petersen, K.F.; LeBon, V.; Enoksson, S.; Ma, Y.-Z.; Savoye, M.; Rothman, D.L.; Shulman, G.I.; Caprio, S. Assessment of skeletal muscle triglyceride content by 1h nuclear magnetic resonance spectroscopy in lean and obese adolescents. Diabetes 2002, 51, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Beasley, L.E.; Koster, A.; Newman, A.B.; Javaid, M.K.; Ferrucci, L.; Kritchevsky, S.B.; Kuller, L.H.; Pahor, M.; Schaap, L.A.; Visser, M.; et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity 2009, 17, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; Di Pietro, L.; Vogel, S.M.; Rothman, D.L.; Shulman, G.I.; Roden, M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1 H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, J.A.; Colberg, S.R.; Thaete, F.L.; Kelley, D.E. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Pollare, T.; Vessby, B.; Lithell, H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler. Thromb. 1991, 11, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nam, S.; Ahn, C.; Kim, K.; Yoon, S.; Kim, J.; Cha, B.; Lim, S.; Kim, K.; Lee, H.; et al. Correlation between midthigh low- density muscle and insulin resistance in obese nondiabetic patients in Korea. Diabetes Care 2003, 26, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A. Muscle Development and obesity: Is there a relationship? Organogenesis 2008, 4, 158–169. [Google Scholar] [CrossRef]
- Wade, A.J.; Marbut, M.M.; Round, J.M. Muscle fibre type and aetiology of obesity. Lancet 1990, 335, 805–808. [Google Scholar] [CrossRef]
- Herbison, G.J.; Jaweed, M.M.; Ditunno, J.F. Muscle fiber types. Arch. Phys. Med. Rehabil. 1982, 63, 227–230. [Google Scholar] [PubMed]
- Tikkanen, H.O.; Näveri, H.; Härkönen, M. Skeletal muscle fiber distribution influences serum high-density lipoprotein cholesterol level. Atherosclerosis 1996, 120, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, H.O.; Härkönen, M.; Näveri, H.; Hämäläinen, E.; Elovainio, R.; Sarna, S.; Frick, M.H. Relationship of skeletal muscle fiber type to serum high density lipoprotein cholesterol and apolipoprotein A-I levels. Atherosclerosis 1991, 90, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L. Sex differences in skeletal muscle fiber types: A meta-analysis. Clin. Anat. 2024, 37, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Nomikos, T.; Kontou, E.; Kiourelli, K.M.; Papadimas, G.; Papadopoulos, C.; Terzis, G. Skeletal muscle fiber composition may modify the effect of nutrition on body composition in young females. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.; Donelan, E.; Abplanalp, W.; Al-Massadi, O.; Habegger, K.M.; Weber, J.; Ress, C.; Mansfeld, J.; Somvanshi, S.; Trivedi, C.; et al. High-Density Lipoprotein maintains skeletal muscle function by modulating cellular respiration in mice. Circulation 2013, 128, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Schisterman, E.F.; Siega-Riz, A.M.; Browne, R.W.; Gaskins, A.J.; Trevisan, M.; Steiner, A.Z.; Daniels, J.L.; Zhang, C.; Perkins, N.J.; et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: Findings from the BioCycle study. J. Clin. Endocrinol. Metab. 2010, 95, E80–E85. [Google Scholar] [CrossRef]
- Calling, S.; Hedblad, B.; Engström, G.; Berglund, G.; Janzon, L. Effects of body fatness and physical activity on cardiovascular risk: Risk prediction using the bioelectrical impedance method. Scand. J. Public Health 2006, 34, 568–575. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Jerosch-Herold, M.; Lima, J.A.C.; Ding, J.; Allison, M.A. Visceral adiposity and the risk of metabolic syndrome across body mass index. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef]


| NW N = 55 | OW N = 149 | OB N = 246 | p-Value | |
|---|---|---|---|---|
| Anthropometric Parameters | ||||
| Age (years) | 28 ± 7 [26, 30] b | 29 ± 7 [28, 30] | 31 ± 7 [30, 31] | 0.031 * |
| Weight (kg) | 63 ± 8 [61, 65] a,b | 77 ± 10 [76, 79] c | 98 ± 15 [96, 100] | <0.001 ** |
| Height (cm) | 165 ± 9 [162, 167] | 166 ± 9 [165, 168] c | 166 ± 10 [165, 168] | 0.398 |
| BMI (kg/m2) | 23.1 ± 1.4 [23, 24] a,b | 27.8 ± 1.5 [28, 28] c | 35.3 ± 4.1 [35, 36] | <0.001 ** |
| Waist Circumference (cm) | 80 ± 6 [79, 82] a,b | 92 ± 8 [91, 94] c | 109 ± 11 [107, 110] | <0.001 ** |
| Body composition | ||||
| R (Ω) | 585 ± 68 [567, 604] a,b | 543 ± 75 [530, 555] c | 474 ± 60 [466, 481] | <0.001 ** |
| Xc (Ω) | 68 ± 8 [66, 70] a,b | 65 ± 8 [64, 66] c | 60 ± 8 [59, 61] | <0.001 ** |
| Φ (degrees) | 6.67 ± 0.80 [6.5, 6.9] b | 6.90 ± 0.87 [6.8, 7.0] c | 7.25 ± 0.91 [7.1, 7.4] | <0.001 ** |
| Vector Length (Ω/m) | 361 ± 53 [346, 375] a,b | 330 ± 55 [321, 339] c | 289 ± 46 [283, 295] | <0.001 ** |
| FMI (kg/m2) | 10 ± 3 [9.2, 11] a,b | 15 ±3 [14, 15] c | 22 ± 6 [22, 23] | <0.001 ** |
| SMI (kg/m2) | 9.88 ± 1.15 [9.6, 10] a,b | 10.59 ±1.49 [10, 11] c | 11.83 ± 1.45 [12, 12] | <0.001 ** |
| Laboratory parameters | ||||
| Plasma Glucose (mg/dL) | 84 ± 8 [82, 86] b | 87 ± 10 [85, 89] c | 90 ± 11 [88, 91] | <0.001 ** |
| Total Cholesterol (mg/dL) | 181 ± 39 [170, 191] | 179 ± 33 [174, 184] c | 190 ± 36 [185, 195] | 0.007 ** |
| HDL-Cholesterol (mg/dL) | 56 ± 13 [52, 59] b | 52 ± 11 [51, 54] c | 48 ± 11 [47, 49] | <0.001 ** |
| LDL-Cholesterol (mg/dL) | 107 ± 31 [98, 115] b | 106 ± 28 [102, 111] c | 116 ± 32 [112, 120] | 0.005 ** |
| Triglycerides (mg/dL) | 92 ± 56 [77, 107] b | 100 ± 66 [90, 111] c | 130 ± 62 [122, 138] | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonardo, M.S.; Guida, B.; Cacciapuoti, N.; Chiurazzi, M.; Pacella, D.; Cataldi, M. Evidence That Skeletal Muscles Modulate HDL-Cholesterol in Metabolic Healthy Young Adults. Nutrients 2024, 16, 1110. https://doi.org/10.3390/nu16081110
Lonardo MS, Guida B, Cacciapuoti N, Chiurazzi M, Pacella D, Cataldi M. Evidence That Skeletal Muscles Modulate HDL-Cholesterol in Metabolic Healthy Young Adults. Nutrients. 2024; 16(8):1110. https://doi.org/10.3390/nu16081110
Chicago/Turabian StyleLonardo, Maria Serena, Bruna Guida, Nunzia Cacciapuoti, Martina Chiurazzi, Daniela Pacella, and Mauro Cataldi. 2024. "Evidence That Skeletal Muscles Modulate HDL-Cholesterol in Metabolic Healthy Young Adults" Nutrients 16, no. 8: 1110. https://doi.org/10.3390/nu16081110
APA StyleLonardo, M. S., Guida, B., Cacciapuoti, N., Chiurazzi, M., Pacella, D., & Cataldi, M. (2024). Evidence That Skeletal Muscles Modulate HDL-Cholesterol in Metabolic Healthy Young Adults. Nutrients, 16(8), 1110. https://doi.org/10.3390/nu16081110






